There is therefore a chronological overlap between the oldest Paranthropes and the most recent Australopithecines, Australopithecus sediba having lived until 1.9 Ma, as well as with the Homo genus, which emerged around 2.8 – 2.5 Ma.
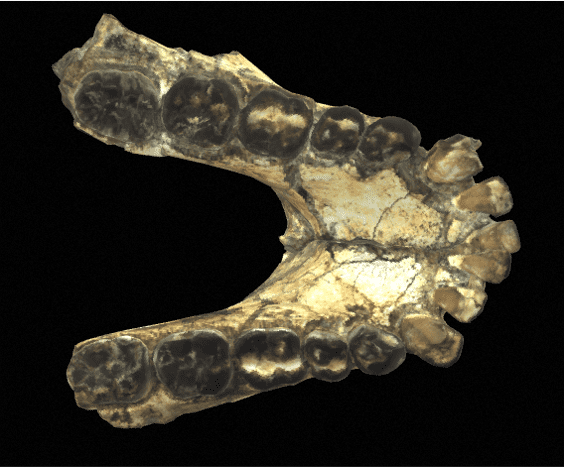
The Paranthropus genus is relatively easy to distinguish from other genera. Indeed, it is characterized by an extremely well-developed masticatory apparatus, with massive mandibles, very large premolars and molars, and very powerful masticatory muscles. In general, Paranthropes are characterized by great robustness.
A little history of science
Before going into more detail on the morphology of these 3 species, let’s take a look at the history of the Paranthropus genus. Indeed, the creation of the latter was not without its difficulties. The genus Paranthropus was first defined in 1938 by Robert Broom, following the discovery that same year of fossil remains at the Kromdaai site near Sterkfontein in South Africa. The adult male skull found was then described as Paranthropus robustus.
A few years later, in 1951, it was suggested (by Washburn and Patterson, to name but two) that the morphological differences observed between the genera Australopithecus and Paranthropus were not sufficient to justify the existence of a second genus, namely Paranthropus. A long debate within the scientific community then began on the scientific legitimacy of the genus Paranthropus.
The heart of the debate then lies in South Africa, where the Australopithecus africanus species is also present. For some scientists at the time, the particularly robust specimens (now P. robustus) were distinguished from the more graceful specimens (nowA. africanus ) also found in South Africa by a question of sexual dimorphism. So, still according to them, it’s a single species, A. africanus, with the robust individuals being the males and the more graceful individuals being the females. However, it soon becomes clear that all the gracile forms come from one and the same site, Sterkfontein, and that the same applies to the robust forms, which all come from the Swartkrans and Kromdraii sites!
What’s more, the fauna present in the Sterkfontein sedimentary fill is older and more archaic than that found at Swartkrans. This variation between the two deposits cannot therefore be the result of sexual variation, but most probably corresponds to species variation. However, some researchers decided to divide all the fossils into 2 species, Australopithecus africanus and Australopithecus robustus.
In East Africa, the first trace of Paranthropes was found in 1955 in Bed II of Olduvai (Tanzania) with the discovery of specimen OH3 (2 teeth, 1 deciduous canine and a molar) by the Leakeys. The taxonomy of this specimen remained uncertain until the 1959 discovery of a skull in Bed I of Olduvai (OH5), again by the Leakeys. For Louis Leakey, this skull differs from both Australopithecus and Paranthropus, creating a new genus, Zinjanthropus boisei. History repeats itself and in 1967, Tobias et al. consider Z. boisei to be an Australopithecus species and rename it Australopithecus boisei. The last Paranthropes species to be recognized was in 1968, under the name Paraustralopithecus aethiopicus (Arambourg and Coppens, 1968), the holotype being an adult mandible discovered in the Omo region (Ethiopia). Paraustralopithecus aethiopicus also became Australopithecus aethiopicus.
Thus, in the 1980s, the 3 current species of Paranthropes all belong to the Australopithecus genus. The name Paranthropus eventually came back into fashion following cladistic analyses demonstrating the monophyly (= 1 common ancestor) of the group formed by A. boisei, A. aethiopicus and A. robustus. It therefore seems more “logical” to group them together and separate them from Australopithecines. According to the principle of anteriority in nomenclature (to find out more, read this article), the name Paranthropus must be included.
Thus, A. boisei, A. aethiopicus andA. robustus are finally attached to the genus Paranthropus. Nevertheless, it should be noted that not all researchers agree with this, and some still use Australopithecus.
Morphological characteristics of Paranthropes
Let’s leave these epistemological debates for the moment and get back to the Paranthropes! These are classified into 3 species with different chronological extensions and geographical distributions:
- Paranthropus aethiopicus was present in East Africa between 2.98-2.87 and 2.3 Ma
- Paranthropus boisei was present in East Africa between 2.3 and 1.3 Ma
- Paranthropus robustus was present in South Africa between 2.2 and 1.2 Ma

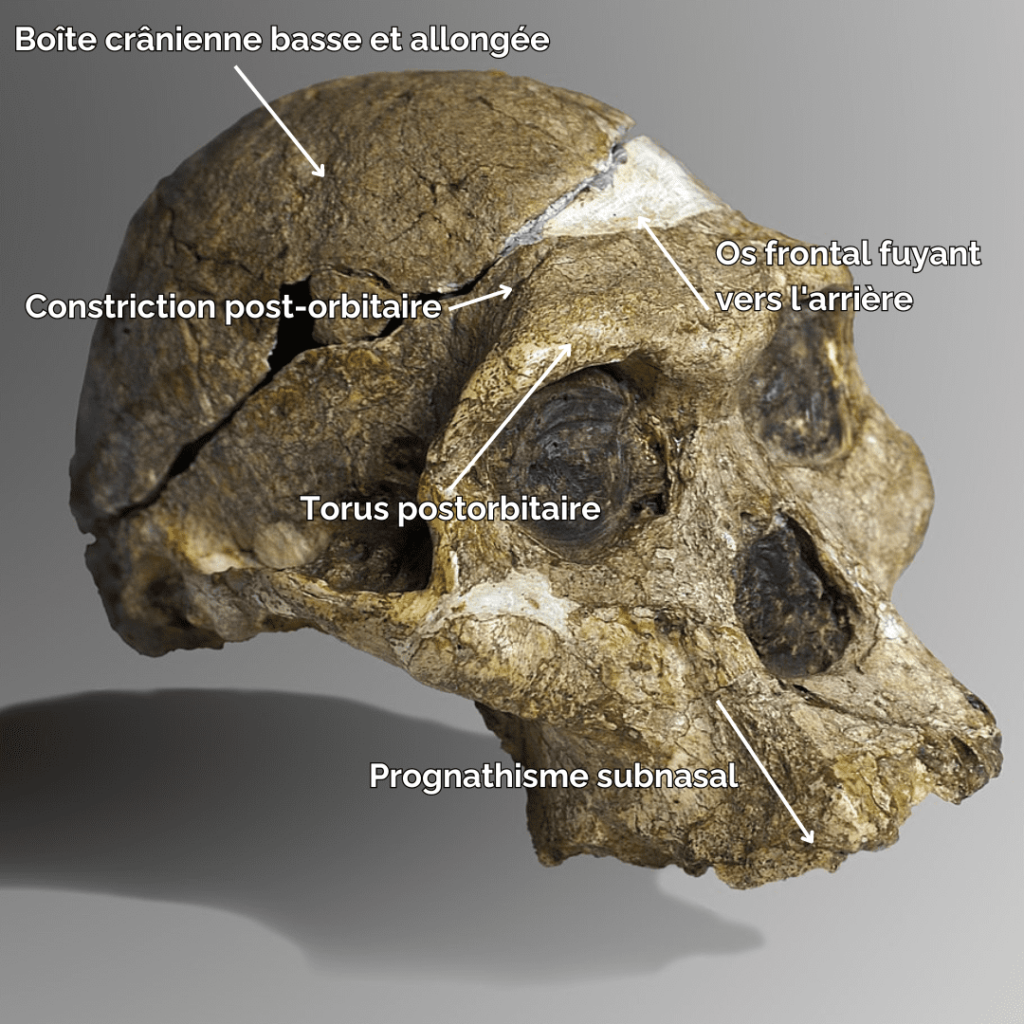
In terms of morphological characteristics, Paranthropes are characterized by extremely extensive development of the cranial superstructures. For example, males of all 3 species have a sagittal crest. The zygomatic arches are highly developed and set back from the skull. The face of Paranthropes is also wide and “hollow”, with significant alveolar prognathism, and they have no chin.

The mandibles are particularly thick and wide (much more so than in Australopithecines), and the molars are very large. Unlike Australopithecines, although they have very large premolars and molars, Paranthrope canines and incisors are small and frontally aligned. As for the skull, it features a strong post-orbital constriction (narrowing of the skull behind the orbits), a low cranial vault, a very narrow frontal bone, a cranial capacity (CC) of between 419-550 cm3 (chimpanzees have a CC of around 500 cm)3) and a thick supraorbital torus.

There are some morphological differences between the three Paranthrope species. However, the distinction between them is also based on geographical areas and chronological extension.
Locomotion & habitats
Another interesting point about Paranthropes is their type of locomotion. It is also accepted that the latter were bipedal, with more efficient bipedalism than Australopithecines, most of whom were still arboreal. Nevertheless, their bipedalism differed from our own, as Paranthropes do not have exactly the same locomotor skeleton as we do. It is also proposed that, like Australopithecines, Paranthropes may have practiced arboricolism. It should be noted, however, that the question of locomotion in this group is highly debated, as very few postcranial remains have been found.
Finally, Paranthropes lived in open (savannah-type) or closed (forest canopy) environments. Contrary to what has long been believed because of their highly-developed masticatory apparatus, Paranthropes’ diet did not consist mainly of tough foods. This belief earned P. boisei the nickname “nutcracker” (OH5). The latter was in fact only an occasional user. In reality, the Paranthropes’ diet consisted mainly of plants.
We hope you’ve enjoyed this introduction to the genus Paranthropus! Feel free to ask us questions and give us feedback on the blog. You can also contact us by e-mail. You can also follow us on Instagram, Facebook, TikTok, Twitter and YouTube!
We would like to thank paleoanthropologist François Marchal for reviewing the first version of this article.
See you soon,
The Prehistory Travel team.
Bibliography :
[1] P. Constantino, B. Wood, “Paranthropus paleobiology”, In: Miscelanea en Homenaje a Emiliano Aguirre. Volumen III: Paleoantropologia, 2004.
[2] D. Grimaud-Hervé et al.Histoire d’ancêtres. La grande aventure de la PréhistoireErrances,5th edition, 2015.
[3] A. Rotman, “The Robust Australopithecines: evidence for the genus Paranthropus”, University of Western Ontario Journal of Anhtropology, 2011.
[4] C. Springer, P. Andrews, The complete world of Human evolution, ed. Thames & Hudson, 2011
[5] B. Wood, Wiley-Blackwell Encyclopedia of Human Evolution, Wiley-Blackwell. Reprinted edition (2013).
[6] B. Wood, P. Constantino, “Paranthropus boisei: Fifty years of Evidence and analysis”, Am. J. Phys. Anthropol. 2007